Healing Innovations: The Future of Advanced Medical Dressings
MEDICAL EQUIPMENT&PRODUCTS
8/17/20254 min read
Introduction: A New Era in Wound Care
Wound care has always been a vital branch of medical science. From the earliest herbal compresses to modern hydrocolloid and hydrogel dressings, each generation of medical products has been designed to protect, heal, and restore. Today, the industry finds itself at the intersection of biotechnology, material science, and pharmaceutical engineering. Advanced dressings such as PU aliphatic foam with active drug release, silicone transparent films, IV infusion patches, and catheter fixation dressings are redefining standards of patient care.
These products are more than passive coverings—they are active, intelligent, and patient-centered. They regulate moisture, release therapeutic agents, prevent infections, and adapt to the natural contours of the body. This article delves into the fascinating world of modern dressings, unpacking their structures, materials, specifications, manufacturing processes, and real-world applications.
PU Aliphatic Foam Dressing: Science Meets Therapy
Among the most innovative wound dressings is the PU aliphatic foam dressing infused with Ibuprofen. It combines moisture management with pain relief, creating a product that accelerates healing while reducing patient discomfort.
Product Features and Dimensions
Available sizes: 5×7 cm, 10×10 cm, 15×15 cm
Annual production target: 750,000 pieces
Structural layers: TPU film + PU foam + release liner
This dressing does not simply cover a wound—it transforms into a micro-environment that manages exudate, delivers pain relief, and minimizes the trauma of dressing changes.
Composition and Active Ingredients
Polyurethane Foam Matrix: Soft, hydrophilic, non-adherent, and highly absorbent.
API (Ibuprofen): 0.5 mg/cm², equivalent to ~60 mg per 10×12 cm piece.
Drug Release Profile: Sustained for up to 7 days, activated by wound exudate.
Backing Layer: Semi-permeable, waterproof TPU film with MVTR ≈ 1800 g/m²/24h.
Adhesive: None—ideal for fragile or sensitive skin.
Additives: Nonionic surfactants as foaming aids.
Clinical Advantages
This design merges pain management with exudate absorption. Instead of prescribing oral analgesics, clinicians can directly target localized pain through controlled Ibuprofen release, reducing systemic side effects.
Packaging
Each piece is sterile, single-use, sealed in polyester/polyethylene foil pouches to guarantee product safety.
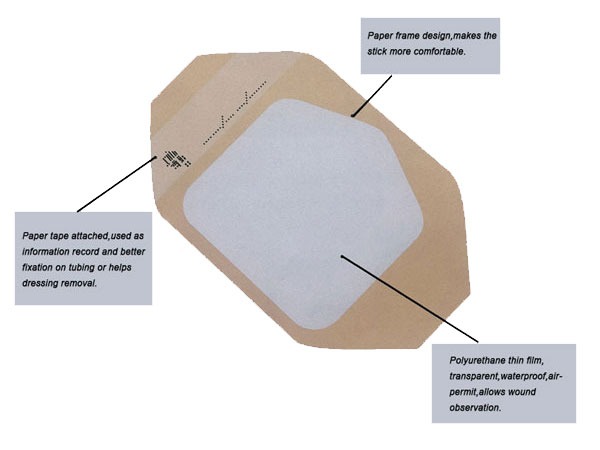
Silicone Transparent Film Dressing: Comfort, Protection, and Clarity
When visibility is as important as protection, silicone transparent dressings step forward. These ultra-thin, body-conforming films act as a second skin, offering flexibility, transparency, and superior barrier functions.
Product Description
Sizes: 6×7 cm, 10×12 cm, 10×25 cm, 15×20 cm
Target production: 17,000,000 pieces annually
Structure: TPU film + silicone adhesive + release liner
Composition and Specifications
Backing Material: Polyurethane film (0.1 mm thick).
Adhesive: Soft silicone—minimizes trauma during removal.
Key Properties:
MVTR ≥ 3,000 g/m²/day
Waterproof, bacterial and viral barrier (>25 nm)
Transparent for wound monitoring
Hypoallergenic and latex-free
Clinical Use Cases
Covering surgical incisions
Protecting IV catheter sites
Preventing infection in minor wounds
Allowing clinicians to observe healing without removal
Packaging
Individually sealed in sterile pouches, ensuring ready-to-use application in both hospital and home-care environments.
IV Infusion Circular Patch: Precision Protection
The intravenous infusion circular patch may appear small, but its impact is significant. Designed to protect puncture sites and catheters, it combines absorbent foam technology with antimicrobial functionality.
Dimensions and Production
Diameters: 2.5 cm (hole sizes: 1.5, 4, 7 mm) and 2.1 cm (hole size: 4 mm).
Annual production target: 370,000 pieces.
Foam and Sponge Specifications
Gelatin Foam: Absorbs up to 60× its weight, with 80–90% retention, thickness 3–6 mm.
PU Foam with CHG: 0.5% chlorhexidine gluconate, open-cell hydrophilic structure, pore size 100–500 μm, density 30–80 kg/m³.
PU Film Properties
Transparent polyurethane with high MVTR, oxygen permeability, tensile strength, and elongation.
Lamination and Manufacturing Process
Prepare PU film
Apply pressure-sensitive adhesive (PSA)
Remove release liner to expose adhesive
Bond gelatin or PU foam base under 5–10 psi
Die-cut to required size and shape
Sterile packaging in medical-grade bags
Packaging
Sealed in medical-grade paper/plastic composite pouches, ensuring sterility and usability.
Catheter Fixation Dressings: Security Meets Simplicity
Catheter stability is crucial for patient safety. Transparent fixation dressings not only anchor catheters but also allow ongoing site monitoring.
Product Range
Transparent fixation dressing with S-shaped release liner (6×7 cm, 7×8.5 cm).
Transparent IV fixation dressing with YAFHO code 623271 (same sizes).
Medical IV transparent dressing, latex-free, with dual securing straps (7×8.5 cm, YAFHO 623277).
CHG-infused IV transparent dressing with antimicrobial pad (7×8.5 cm).
Structural Layers
Outer Film: Medical-grade aliphatic TPU, waterproof and breathable.
Adhesive: Acrylic pressure-sensitive adhesive.
Pad (Optional): Cross-linked PEG hydrogel, PU foam, or hemostatic sponge with CHG.
Application Frame 1: Coated paper.
Application Frame 2 (Optional): Nonwoven coating.
Release Liner: Coated release paper.
Packaging
Delivered in sterile, medical-grade pouches, suitable for clinical use.
Manufacturing Standards and Quality Control
Each of these products must meet ISO 13485 medical device standards and CE certification requirements. Quality checks involve:
Sterility assurance level (SAL) validation
Cytotoxicity, sensitization, and irritation testing
Shelf-life stability studies
Mechanical testing for tensile strength, adhesion, and absorption
Applications in Healthcare Settings
These dressings are deployed in a variety of clinical settings:
Hospitals: Surgical wards, emergency rooms, ICUs.
Home Healthcare: Chronic wound management.
Specialty Clinics: Diabetic foot treatment centers, oncology units.
Military and Disaster Relief: Rapid-deployment kits for field hospitals.
Market Relevance and Global Demand
The demand for advanced wound dressings continues to rise due to:
An aging population with chronic wounds.
Increased surgical procedures worldwide.
Growing awareness of infection prevention.
Technological advances in drug-delivery dressings.
With production targets in the millions of units annually, these products are set to dominate the wound care market.
Conclusion: A Vision for Smarter Healing
The evolution of dressings shows how far medicine has advanced. No longer passive coverings, today’s dressings are dynamic healing platforms—delivering drugs, preventing infection, and improving patient comfort. Whether it’s a PU foam dressing with Ibuprofen, a silicone transparent film, an IV infusion patch, or a catheter fixation dressing, each product is designed with science, safety, and patient well-being in mind.
For healthcare providers, hospitals, and distributors, these innovations represent not just medical tools but strategic assets in improving outcomes and reducing healthcare costs.
Contact Information
For detailed technical and commercial proposals, distribution inquiries, or supply chain discussions, please contact:
LeonWholesale
📞 WhatsApp: +8618136773114
📧 Email: leonxu0317@gmail.com
Hashtags
#MedicalDressings #AdvancedWoundCare #PUFoamDressing #SiliconeFilmDressing #IVPatch #CatheterFixation #WoundManagement #LeonWholesale
